Heterotrigona itama (Cockerell 1918)(Hymenoptera: Apidae: Meliponini)

Figure 1. Side view of Heterotrigona itama. © 2014 S. X. Chui.
Overview l Morphology l Foraging l Nesting & Reproduction l Predators l Defensive Strategy l Distribution l Status in Singapore l Conservation l Taxonomy & Phylogeny l Type Information l References
Overview return to top
A lesser known group of eusocial bees when compared to the ubiquitous honeybees, stingless bees are a pantropical group, unlike honey bees which are only found in the Old World. However, in terms of species numbers, while there are only 11 species of honeybees in a single genus, Apis, there are at least 600 species of stingless bees from some 60 genera.[1] Stingless bees may be relatively inconspicuous, but they do play an important role as pollinators in tropics. Together with honeybees, they are the most abundant pollinators in the lowland dipterocarp forests in Southeast Asia. However, as key pollinators in agriculture in the western hemisphere, and in the production of honey, honeybees have been well studied as compared to stingless bees. Perhaps because of the much higher diversity of stingless bees, there has been a shortage of studies of the biology and ecology of most species in the tribe Meliponini. Compounded with the comparative lack of researchers working on stingless bees in the Southeast Asian region as compared to the New World, there is scant information on most stingless bees in the region beyond a species description.
The aim of this page is to shed some light on Heterotrigona itama (fig. 1), a species which can be found in Singapore. The page targets insect enthusiasts as well as tertiary students and above in the field of biology, and the author hopes that this page has succeeded in linking various aspects of biology and ecology for a more comprehensive understanding of the interactions between this species and its environment.
Morphology return to top Diagnosis

Figure 2. Comparison of 4 common bees. From left: Apis cerana, Heterotrigona itama, Tetrigona apicalis, Tetragonula laeviceps. © 2014 S. X. Chui.
Heterotrigona itama can be easily distinguished from other commonly encountered stingless bee species by size and coloration. It is the largest common species in Singapore, besides Tetrigona apicalis. Both H. itama and T. apicalis have a black body, but unlike T. apicalis which has split colored wings, with the wing apex region white, H. itama has a uniform sepia tinged wing. Tetragonula laeviceps is noticeably smaller than either species. On the other hand, H. itama is smaller than Apis cerana (Asian honeybee). An easy way of distinguishing stingless bees from other bees is by the position of their hind legs while in flight. While other bees that fold their hind legs beneath the abdomen while in flight, the hind legs of stingless bees hang limply downwards.
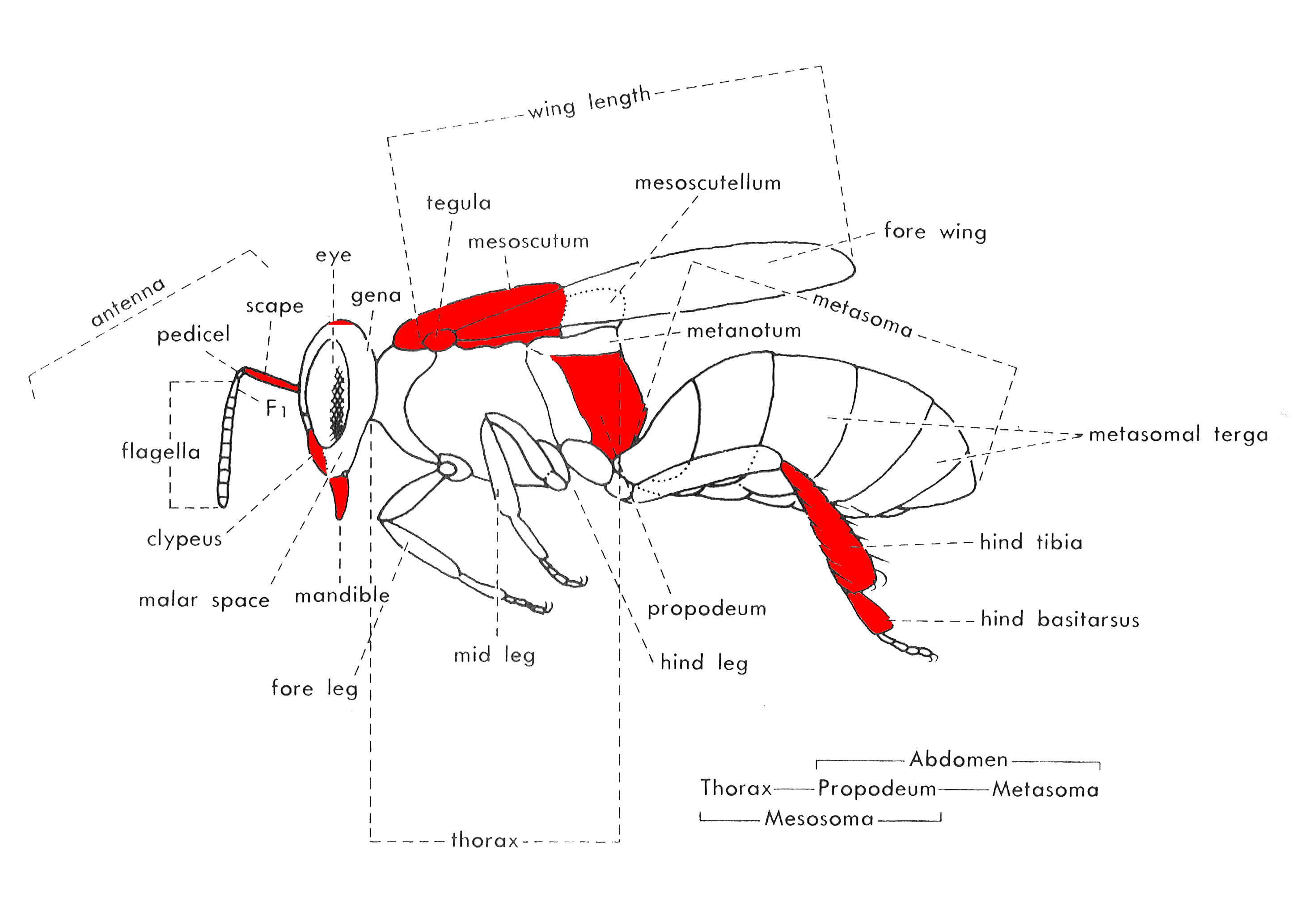
Beyond the straightforward separation of Heterotrigona itama from other locally common stingless bees in Singapore, diagnosis of the species is done through examination of morphological traits of both the workers and males of the species. This page only covers the diagnosis of the species through examination of the worker. Parts of the body important for diagnosing the species are highlighted in red (fig. 3), and are usually examined with the aid of a hand lens or dissecting microscope.
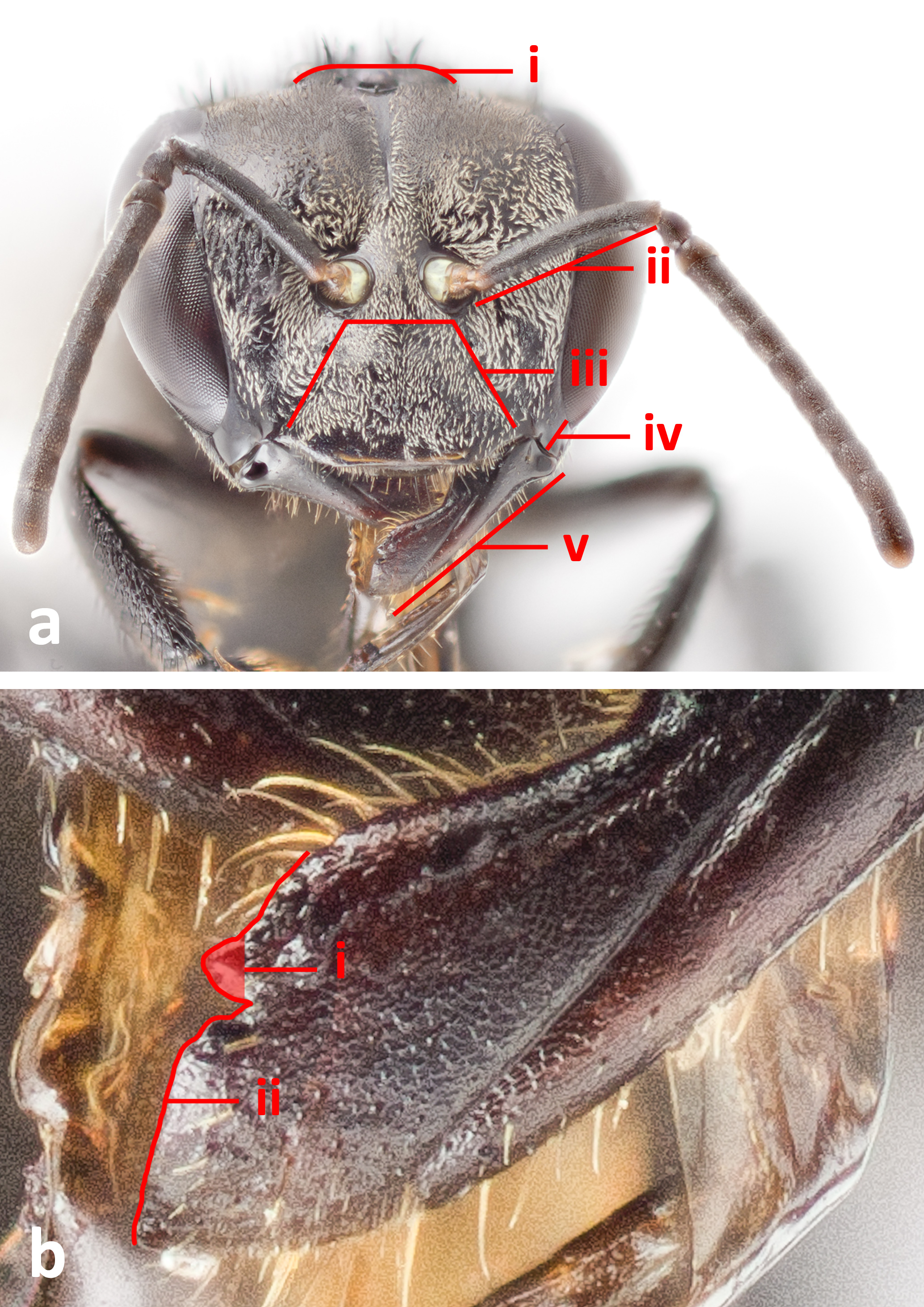
An easy way to narrow down an unidentified specimen to the genus Heterotrigona is through the examination of its mandibles. Species in the genus Heterotrigona have a single tooth (fig. 4bi) on the inner edge of the otherwise edentate mandible apex (fig. 4bii).
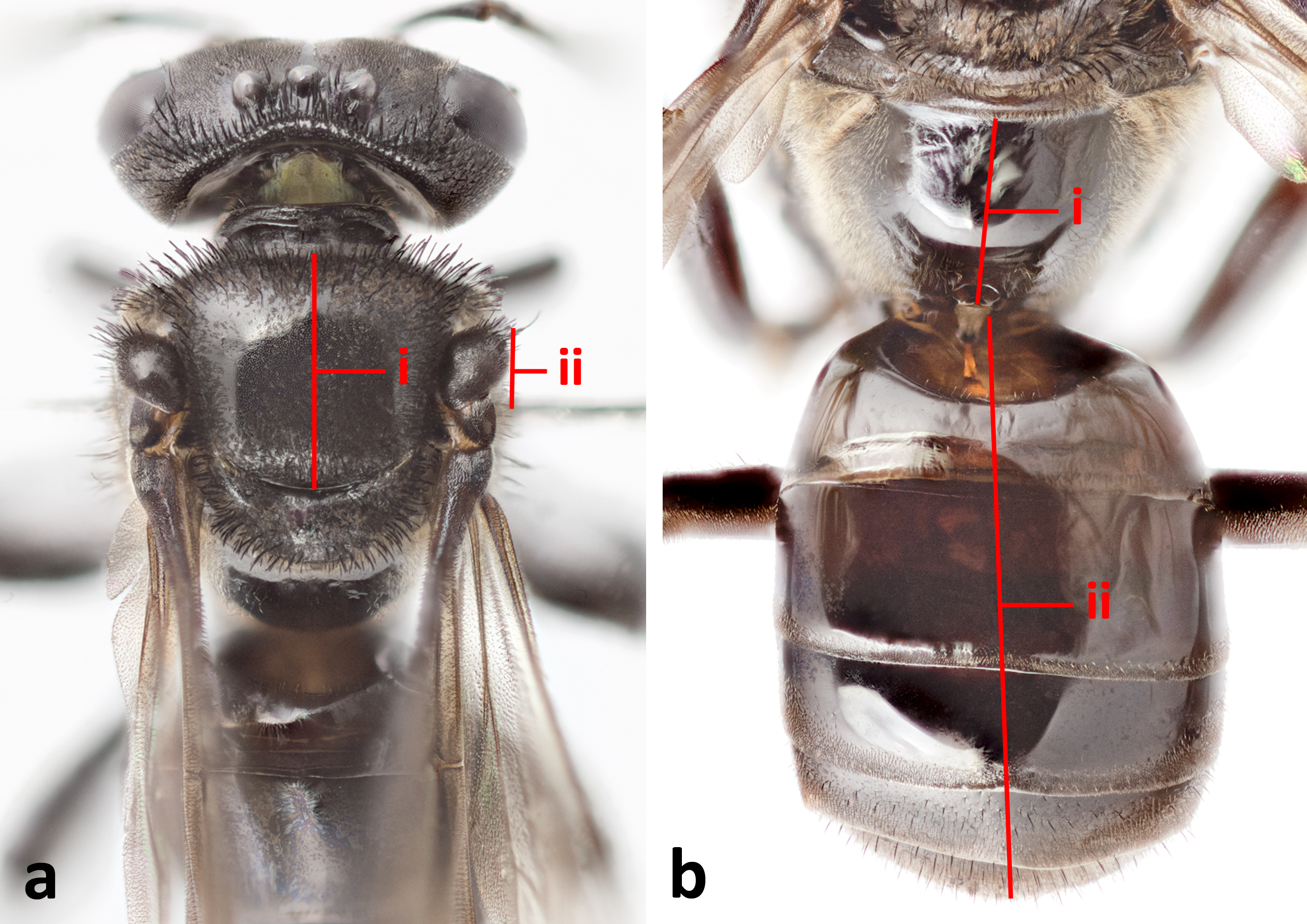
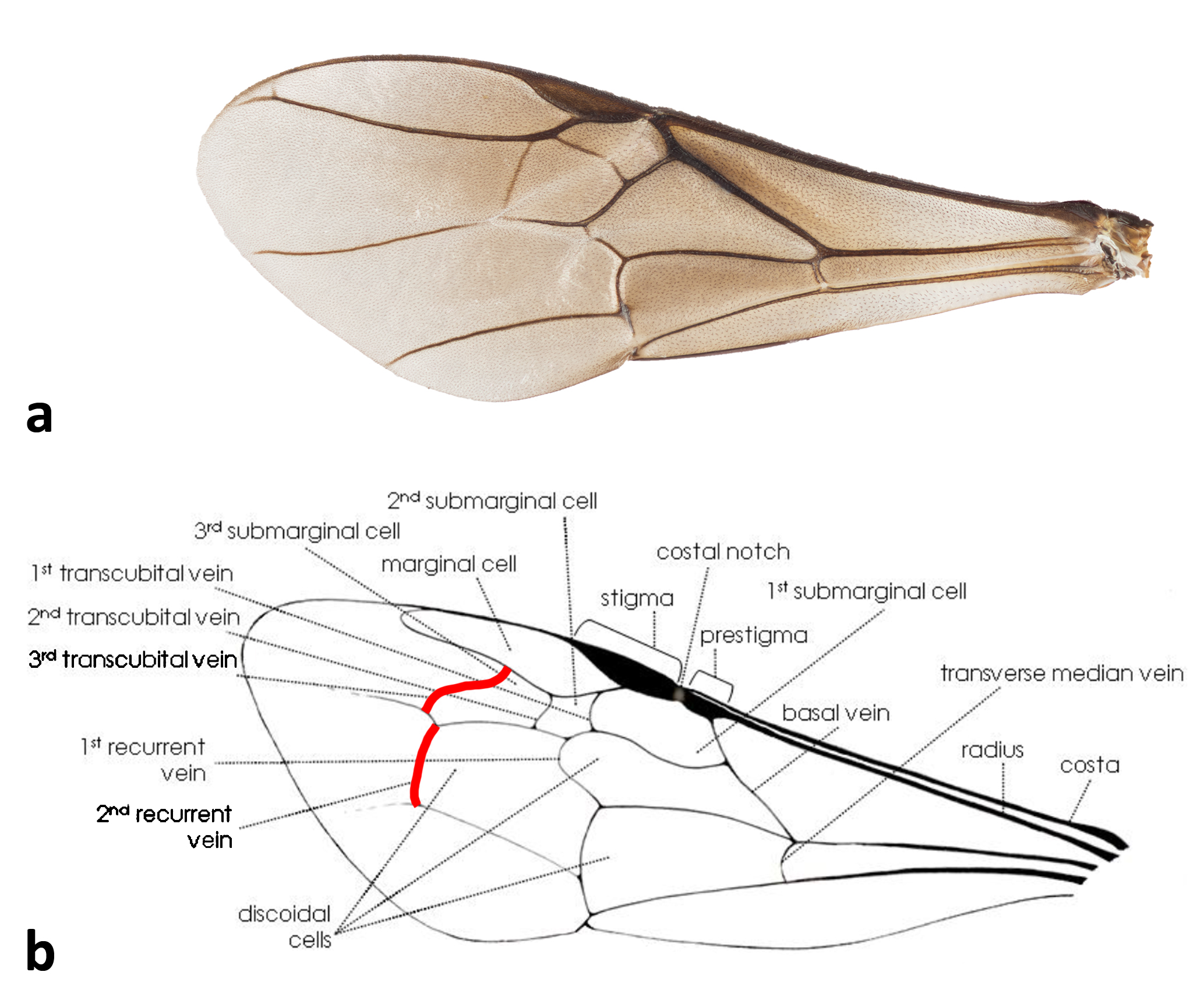

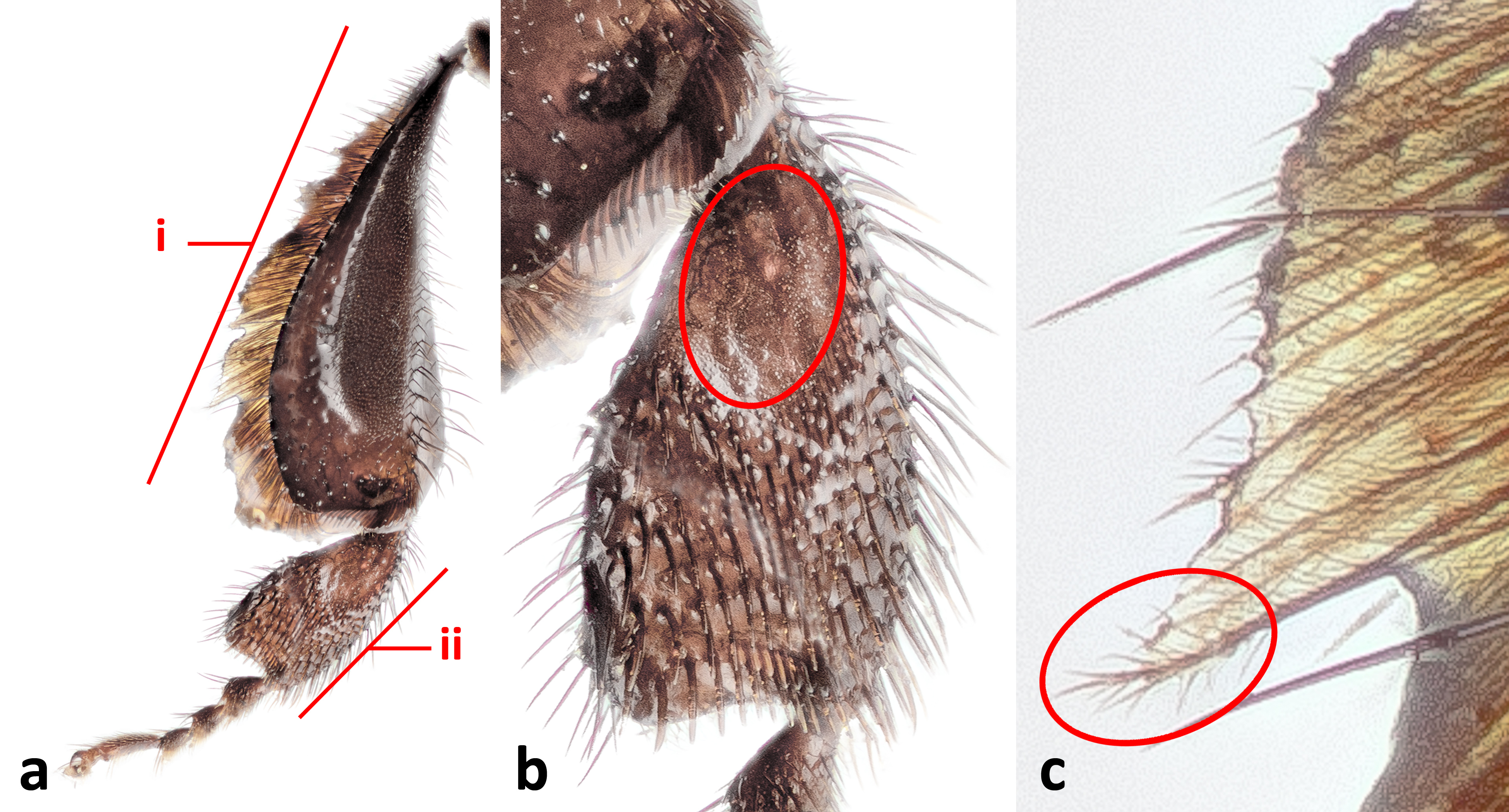
Figure 8. Hind leg of H. itama. 8a: Posterior view of hind leg. i= tiba, ii= basitarsus; 8b: Posterior view of basitarsus. Hairless patch circled; 8c: Closeup of fringe hairs on hind tibia. Note example of plumose hair circled. © 2014 S. X. Chui.
With reference to figures 4-8, and diagnostic features adapted from Schwarz, 1937[3] . here is a summary of morphological characters which define Heterotrigona itama:

Corbiculate bees
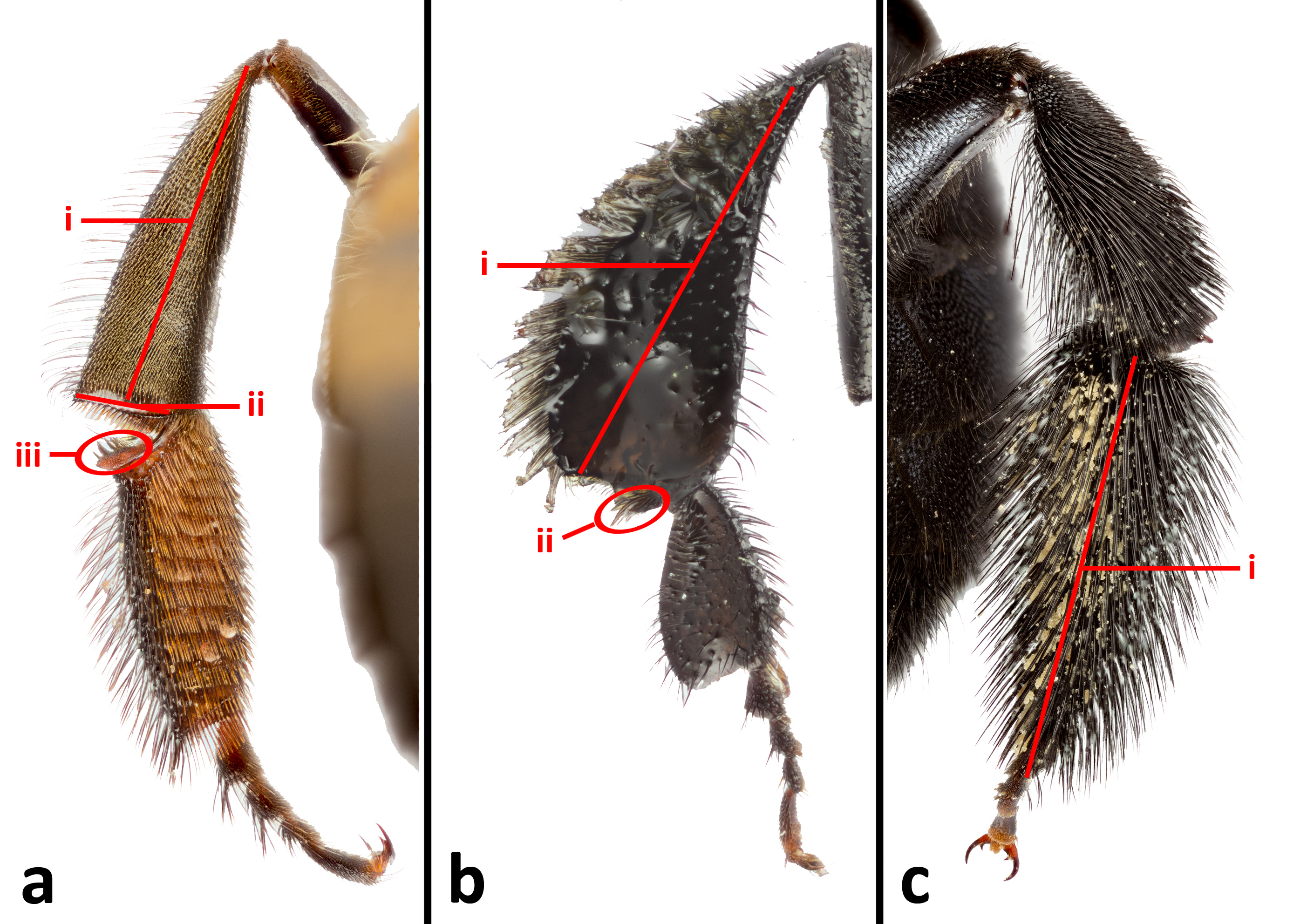
Figure 9. Hind leg comparison of three bees. 9a: Apis dorsata. i= corbicula, ii= rastellum, iii= auricle; 9b: H. itama.
i= corbicula, ii= penicillum; 9c: Xylocopa aestuans. i= scopa on hind basitarsus. © 2014 S. X. Chui.
Bees with corbiculae (pollen baskets) belong to the subfamily Apinae, under the family Apidae. Eusociality is a shared trait of corbiculate bees, namely the four tribes: Apini (honey bees), Bombini (bumble bee), Euglossini (orchid bee), and Meliponini (stingless bee). Corbicula refers to a hairless patch on the outer surface of the hind tibia (fig. 9ai, 9bi), where collected pollen is stored on the bee between foraging and returning to the nest. In non-corbiculate bees, hairs on the hind leg (fig. 9ci) or on the underside of the abdomen are used to trap pollen (scopa). The hairless surface of the corbicula allows for quicker pollen load removal from the bee as compared to bees with scopas. In addition to the corbicula, corbiculate bees also have additional structures which facilitate the packing of pollen before storage on the corbicula. Both stingless bees and honey bees have a rastellum (pollen rake) (fig. 9aii) on the hind tibia, but while both groups have a pollen press on the hind basitarsus, stinglees bees have a relatively weakly developed pollen press when compared to the expanded pollen press (auricle) (fig. 9aiii) in honeybees. However, unique to stingless bees are a tuft of hairs at the joint of the tibia and basitarsus called the penicillum (fig. 9bii). The penicillum was postulated to guide pollen towards the corbicula.
Foraging return to top
Stingless bees forage extensively and effectively with the corbiculae that they possess. Resources foraged for include pollen, nectar, and resin. Stingless bees are resource generalists rather than specialists, which is borne out of necessity given the conditions found in tropical lowland dipterocarp forests.Temporal nature of resource availability
Stingless bees utilise pollen and nectar exclusively as food resources, and resin as a resource for nest construction. Pollen and nectar, as resources sourced from flowers, are ephemeral. Individual plants do not flower constantly, and plant species need not flower sequentially throughout the year. Though stingless bees are found in both paleotropics and neotropics, the paleotropics, in particular the lowland dipterocarp forest of South East Asia present a unique problem. The lowland dipterocarp forests undergo synchronised mass blooming, known as masting, supra-annually. In what is hypothesized to be predator saturation, many species, not confined within the Dipterocarpaceae, produce countless flowers that satiate flower and fruit predators, ensuring the survival of adequate numbers of seeds. The onset of masting seems to be triggered by a prolonged period of dryness, an atypical event in a region where regular precipitation is the norm.[4] The dry period is a result of the El-Nino Southern Oscillation (ENSO) cycle, which spans from South America, across the Pacific ocean, to Southeast Asia. The ENSO cycle occurs every 3-5 years.[5]Outside of masting events, flowering in lowland dipterocarp forests is both spatially and temporally sporadic. Lucrative spots of flowers appear for a brief period, sporadically, and may be separated by considerable distances in the forest. The result of the aforementioned conditions in lowland dipterocarp forests is a surprising paucity of bee species, which contrasts with the great array of flora diversity that these forests are known for.[6] Solitary and and eusocial bees differ in their methods of coping with these conditions. Solitary bees tend towards seasonal cycles, with their populations rising and ebbing with resource availability. Stingless bees, like other eusocial bees in the tropics, cope with fluxes in resource availability by stocking up on resources when they are available, which are stored in their nests for use in lean times.

Effectiveness as pollinators
Stingless bees may visit the flowers of many species of plants, but their effectiveness as pollinators remains poorly studied. Figure 10 shows various forms of flower visitations. The first shows visitation without the possibility of pollination. The stigma is situated far beyond the main flower axis that the Heterotrigona itama pictured here will never contact the stigma. The second shows the opportunistic nature of stingless bee foraging. H. itama was observed to drink from flower stalks that have had freshly fallen flowers from Tabernaemontana divaricata which has otherwise unreachable nectaries for H. itama. The third shows an open flower type that has a greater likelihood of effecting pollination from H. itama. The anthers and stigmas are positioned are approximately the same axis, easily contacting H. itama when it perches within the flower when drinking nectar from the flower. Some stingless bees are also known to engage in nectar robbing, by chewing their way through the base of the flower where nectaries are often concealed.Stingless bees forage from a central point (their nest) in what is known as the central place foraging theory, outwards in a ring to the extent of their maximum flight distance. This contrasts with traplining bees, which move between different flower patches sequentially. Traplining allows for greater transference of pollen between patchily distributed plants in the forest. Stingless bees, with their central foraging, would mean that pollen that collects on the bee from a flower visit has a lower chance of reaching flowers in a different locality as the bee returns to the nest after each short foraging trip. However, as social bees are most numerous in the forest, stingless bees, through their countless interactions with flowers, are still likely to play a substantial role as pollinators for the majority of plants in the forest, as evidenced by the percentage (32%) of species in lowland dipterocarp forests reported to be pollinated by social bees.[7]
Nesting & Reproduction return to top
Nest

During the day, there is a hive of activity at the nest entrance as Heterotrigona itama workers head to and from the nest, returning with pollen, honey, and resin (fig. 11). The nest entrance is guarded by workers, a few of which remain in the entrance tube with their heads oriented towards the exit, while others line the immediate vicinity of the exit. The guards in the immediate vicinity of the nest entrance do not move about much, often beating their wings in short bursts. Occasionally, a guard would approach and investigate the camera setup, of which the front end of the lens was approximately 20cm from the nest entrance.
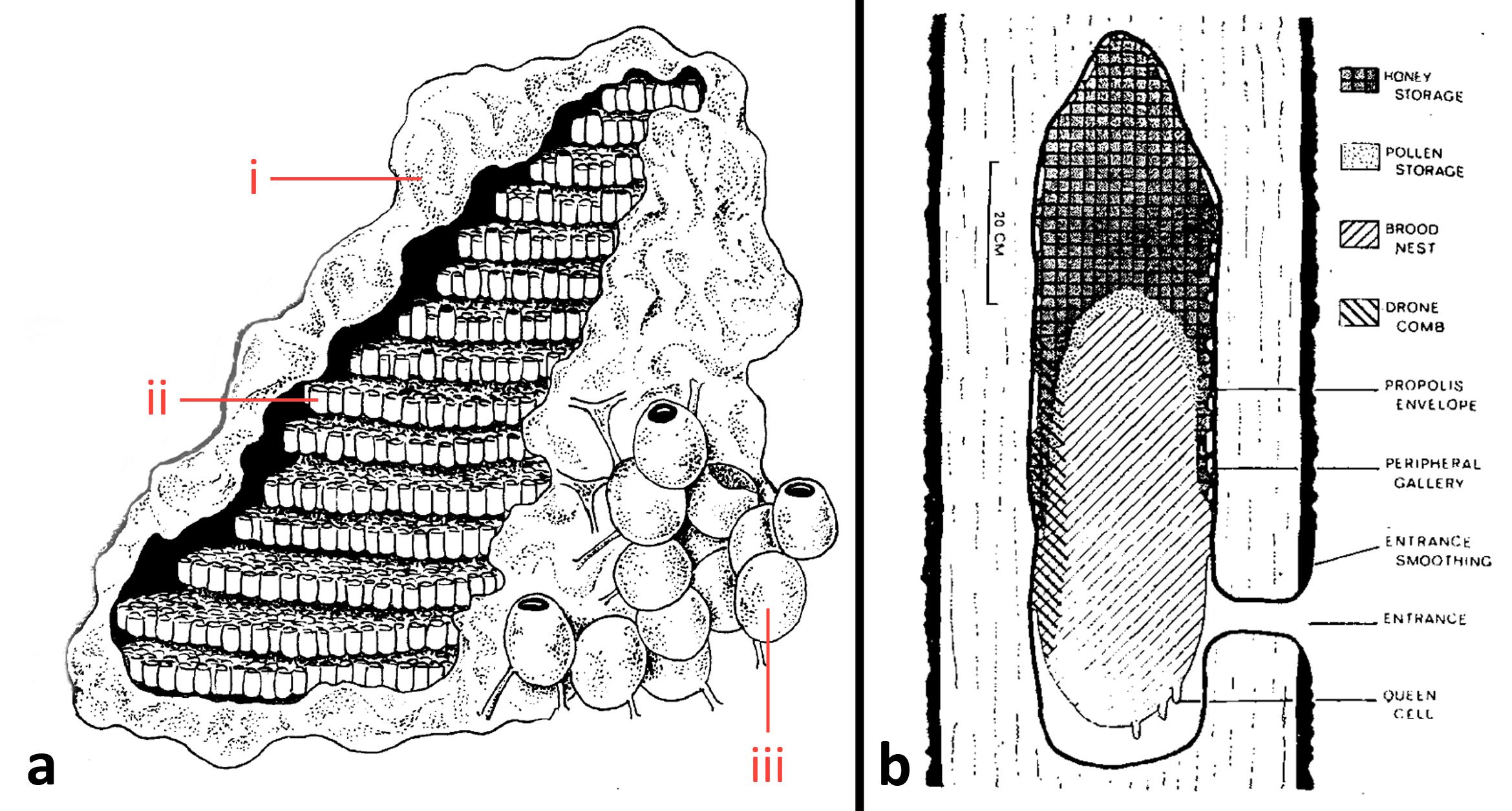
Heterotrigona itama nest in trunk hollows, with an entrance tube made of cerumen often protruding beyond the surface of the trunk. H. itama constructs different cell types for different functions. Brood cells (fig. 12aii) of H. itama form a horizontal comb that is separated from the previous comb by support pillars. The brood combs are enveloped in involcrum (propolis) (fig. 12ai). Honey and pollen pots (fig. 12aiii) are an upright elliptical shape, with an opening at the top of pots that are currently in use. In contrast, honeybees construct their wax combs in a vertical orientation (fig. 12b). Unlike H. itama which partitions resources by cell type, honeybees utilise a single hexagonal cell design for both the rearing young, and storage of pollen and honey.
Establishment of new nests
Several times a year, a mature nest produces queens and drones in anticipation of nest establishment. The process of establishing a new nest starts with scout bees, which search for suitable nest sites. New nests are dependent on mature nests for initial establishment, with workers gradually transporting nesting materials, pollen, and honey to the new nest over an extended period of time. Swarming occurs when a virgin queen leaves the mature nest for the new nest with a swarm of workers, from which a portion of older workers will stay with her. Within two days of arrival, the virgin queen proceeds to make her nuptial flight, surrounded by drones that vie to mate with her, where she mates with only one drone. After mating, oviposition commenced 5-6 days after the initial arrival of the queen to the new nest.[10]Comparison with honeybees
In contrast with stingless bees, establishment of new nests is an abrupt affair.[11] During swarming, swarms sever ties with the old nest and set off to establish new nests independent of the old one. Unlike stingless bees where the original queen of the mature nest retains control, in honeybees the original queen swarms with a mass of workers and leaves to establish a new nest. Virgin queens from subsequent swarms, as well as one that will replace the original queen of the mature nest, perform nuptial flights where they mate with multiple drones. In both stingless bees and honeybees, the queen mates only once as she is able to store a lifetime's supply of sperm in her spermatheca (sperm storage organ).Kin selection
Kin selection, well studied in eusocial bees, is present in both stingless bees and honeybees. However, intra-nest dynamics between the different castes (queen, virgin queens, workers and drones) differs between stingless bees and honeybees.[12] Mate number has been suggested as the cause of downstream differences after a virgin queen mates. In stingless bees, workers are more related to each other, and their nephews (drones), than they are to the queen, and thus workers have a higher tolerance of each other laying unfertilised eggs (drones) in cells on occasion. In honeybees, workers are more related to the queen than they are to each other, and thus do not tolerate the survival of drones produced by workers. A result is that stingless bees often have drones that do not originate from the queen.Rearing of young
To feed their larvae, stingless bees practise mass provisioning, in contrast with honeybees which constantly feed their young. Mass provisioning is a trait that stingless bees share with most solitary bees. In mass provisioning, pollen and nectar is mixed to form 'bee bread', which is deposited in empty cells, followed by the laying of a single egg in the cell. The cell is then sealed and the larvae which hatches proceeds to develop, living off the food in the cell, pupate, and then emerge from the cell as an adult.[13] The rearing of queens in stingless bees is determined by the amount of food that is provided to the larvae, unlike honeybees where 'royal jelly' is the determinant of queen rearing.[14] Workers and queens develop from fertilised eggs, while drones develop from unfertilised eggs.Predators return to top
As a eusocial species, stingless bees face threats at the individual level, as well as at the nest level. The destructive ability of the predator is dependent on its size. Macro-predators of stingless bees within Southeast Asia include sun bears and primates such as orang utans, which have the ability to destroy the outer part of the tree trunk to gain access to the nest, in order to reach the honey stores.[15] [16] Micro-predators of individual stingless bees include assassin bugs (Reduviidae), some of which are convincing mimics of stingless bee species[17] .
Defensive Strategy return to top
The common name, stingless bees, is a misnomer and often leads to the misperception that these bees do not have stings. Partly true, stingless bees do have stings, but are greatly reduced and are unable to penetrate human skin. One would be mistaken to think that stingless bees are defenseless without their stings. Their defense strategy can range from retreating into the nest, to biting, spitting out acid.[18]Heterotrigona itama was observed to have fully extended mandibles when the nest was provoked.[19] The 'call to arms' alarm pheromone is instrumental in coordinating defense.[20] Individual stingless bees latch on to exposed hair, and try to tunnel as deeply into the mass of hair as possible. The entrenched bee releases an alarm pheromone that alerts others in the nest that the nest has to be defended. Removal of the pheromone from the vicinity halts defensive behavior on the part of the bees.Function of the sting apparatus in stingless bees
The sting is clearly non-functional for stinging as the stylet of the stinger is much reduced, but it is not without an alternative function. Unlike other aculeate Hymenoptera with a concave gonostylus (protects stylet), the gonostylus of stingless bees is convex, with the presence of receptor hairs on the apical portion[21] . The presence of the receptor hairs, and the convex shape of the gonostylus, led to the hypothesis that the gonostylus in stingless bees functions as a mechanoreceptor that triggers a defensive response by the individual bee. Upon contacting non-sclerotized surfaces (eg. skin) with the gonostylus hairs, the bee is induced to bite with its mandibles, as in the case of Heterotrigona itama.Distribution return to top
The distribution of a species is influenced by a host of factors, which include some of the preceding sections of this page. Factors include: place of origin, ability to disperse, adaptability to local conditions (foraging, nesting), and changing geographies. Only by understanding these various aspects can one hope to have a clearer understanding of the present distribution of a species.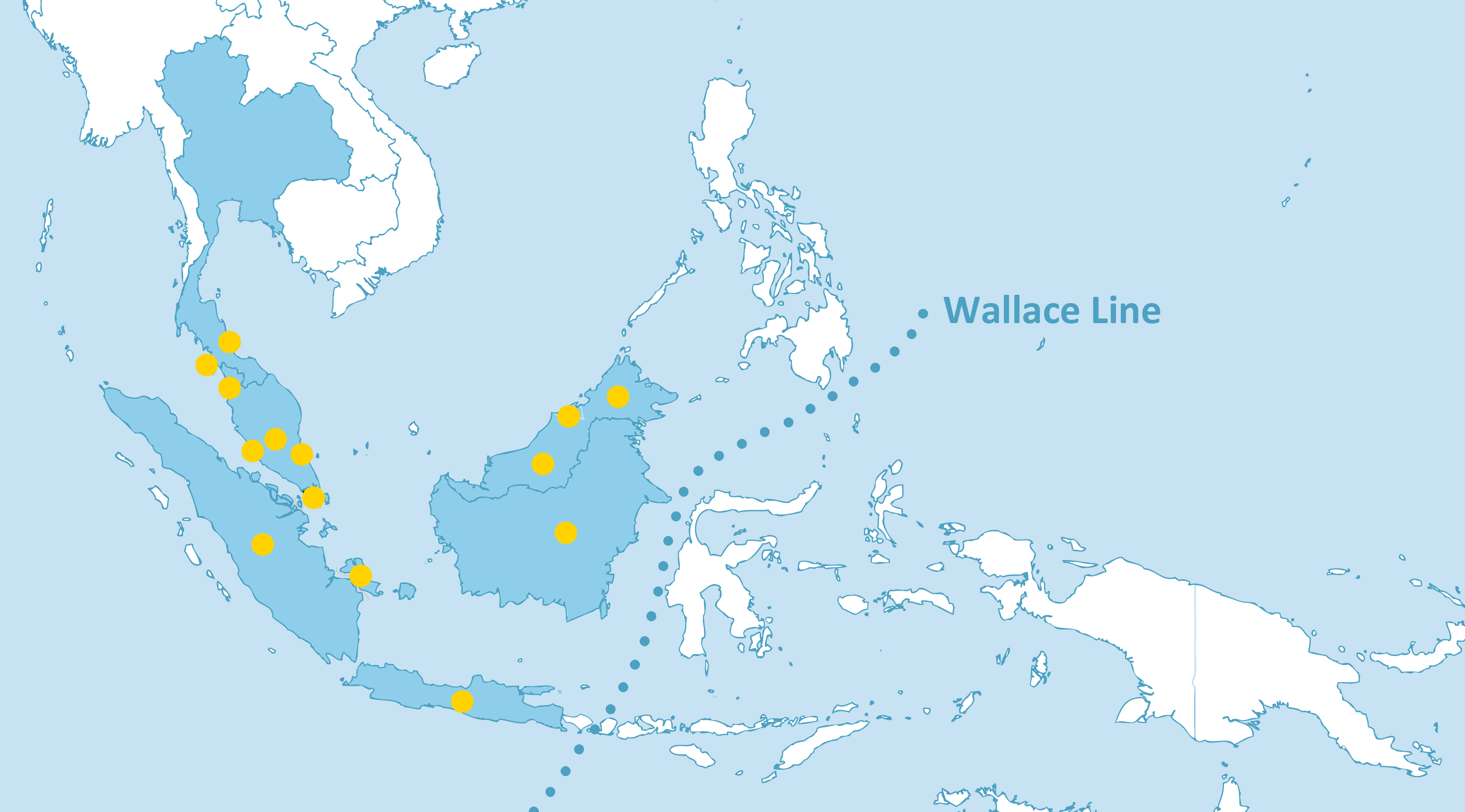
H. itama is distributed within Sundaland (fig. 13), ranging from Thailand in the north to Java to the south, and Borneo to the East.[22] The habitat of this species is lowland tropical dipterocarp forest.
Ability to disperse
Heterotrigona itama, like other stingless bees, are not able to disperse over great distances or over large stretches of water, for which there are three reasons.[23]Firstly, the inability of stingless bees to fly long distances, as demonstrated in a study on the correlation between body size and flight distances in stingless bees.[24]
Secondly, as a eusocial species, a hive of stingless bees constitutes a single reproductive unit. A nest, though consisting of numerous workers, usually only contains a single queen. This is unlike non-eusocial bees, where every female has an equal ability to disperse and reproduce. Thirdly, unlike honeybees, the other major group of eusocial bees, where the queen and a portion of workers abruptly break from the hive on a permanent basis, stingless bees require the continued support of the mother nest for resources for a period after the initial establishment of a new nest.[25]
The third reason plays a substantial role in the dispersal distance of the species. Compared to a possibly farther once-only distance that honeybees undertake, stingless bees are unable to expend such energy if multiple trips between old and new nest are required.
Changing geographies
If stingless bees do not have the ability to cross large stretches of water, how did they disperse within the southeast asian archipelago? By looking into relatively recent geographic history, it is possible to examine a possible reason for the present distribution. In the most recent ice age at the end of the Pleistocene (ca. 10,000bc), sea levels fell by up to 120m, revealing land bridges (between 75-120m below sea level) that connected present day peninsular malaysia to Borneo, Sumatra and Java to form the landmass Sundaland.[26]However, separated by Wallace's Line, islands to the east of Borneo such as Sulawesi, were never connected by land to Sundaland (fig. 13). Heterotrigona itama is likely to have dispersed within Sundaland up till the end of the last ice age, and have been confined to various geographic areas in Southeast Asia since then.
Suitable nesting sites
Unlike honeybees, stingless bee species nest mostly in the trunk hollows of living trees, between tree roots, or in the nest of other invertebrates such as ants and termites.[27] H. itama nests in trunk hollows. H. itama, as a medium sized stingless bee species, requires a proportionately larger hollow to nest in as compared to a smaller species such as T. laeviceps, a locally common species. In a study, nest trees for stingless bees in Sabah, Malaysia were large, with 86.1% of trees above 60cm trunk diameter.[28] For a sustainable population, H. itama needs forest with a number of large diameter trees in which hollows can form. Essentially, good forest.Competition
Stingless bees face inter-specific competition for resources. Resources are in the form of pollen, nectar, and resin. With reference to the competitive exclusion principle, no two species can share the same ecological niche. Stingless bee species such as Tetragonula laeviceps forage aggressively, recruiting numerous workers to lucrative resources, potentially excluding other species from the resource. A suggested reason for the coexistence of multiple species utilizing the same resource is niche preemption, which postulates that a single resource can be split both spatially and temporally into multiple niches.[29] Stingless bee species have differing maximum foraging distances, and individual colonies discover the presence of transient, patchy resources independently of each other, thus allowing for spatial and temporal niches.Status in Singapore return to top
Heterotrigona itama has been observed from selected sites in Singapore. To date, this species has been found in the Singapore Botanic Gardens, Orchard, as well as in the Central Catchment Nature Reserve (CCNR). However, at other green spaces, such as parks, the presence of H. itama has yet to be observed. The seemingly sporadic distribution of H. itama does not indicate the decline of the species in Singapore, but does indicate that more sampling is required before conclusions can be made. However, it is telling from the lack of this species from parks, which do not have stands of mature trees with a sufficient trunk diameter, that the reduction of forest habitats has had a negative effect on suitable nesting sites for H. itama. This in turn influences their local distribution. It is unknown if the population in Singapore is viable in the long term.Of the 14 stingless bee species ever recorded in Singapore, 6 have been found by the author. An interesting common feature of the 6 species is their relatively small size, with H. itama and Tetrigona apicalis the two largest local species (body length ~ 6mm). The likely decline of the local number of stingless bee species, and the size of species still present, speaks of increasing pressures on stingless bees in Singapore. This contrasts with data on solitary bees and honeybees, which demonstrates that the diversity of solitary bee species and honeybees is still consistent between past and present. It can only be guessed as to why there exists such a contrast in local population survival between stingless bees and solitary/honey bees.
Stingless bees, active all year round, face a decreasing abundance of available resources in Singapore with increasing forest reduction and fragmentation.[30] Larger species require correspondingly larger cavities to nest in, which translates to the requirement for trees with larger trunk diameters. Also, as eusocial insects, stingless bees function as a single reproductive unit. While the majority of encountered bees in Singapore forests were from the Apidae (honey bees, stingless bees), the actual number of reproductive units are far fewer than the numbers of bees encountered or captured.[31] Whether the small forest fragments within a developed city state such as Singapore retain the capacity to sustain viable populations of stingless bees is a cause of concern.
Conservation return to top
With the abundance of flowers at forest edges, native and exotic, wild and planted, the sustainability of a viable population of Heterotrigona itama is Singapore is not limited by the lack of food. The limiting factor is likely to be the availability of suitable nest sites. One suggestion that will increase the availability of nest sites is to supplement natural tree hollows with constructed nest boxes, attached to trees in the forest, hopefully increasing the odds of the survival of the Singapore population.[32]Stingless bee apiculture
A logical progression of providing nest boxes in the forest for stingless bees is the commercial application of apiculture (beekeeping). Apiculture of stingless bees, while not practised in Singapore, is common in neighboring countries such as Malaysia and Indonesia. Stingless bees have the ability to produce good yields of honey, which can be used to supplement a family's diet or sold for profit. Do note however, that not all stingless bees honey is fit for safe consumption.Taxonomy & phylogeny return to top
Taxononavigation
Kingdom: AnimaliaPhylum: Arthropoda
Class: Insecta
Order: Hymenoptera
Superfamily: Apoidea
Family: Apidae
Subfamily: Apinae
Tribe: Meliponini
Genus: Heterotrigona
Specific epithet: itama
Phylogeny
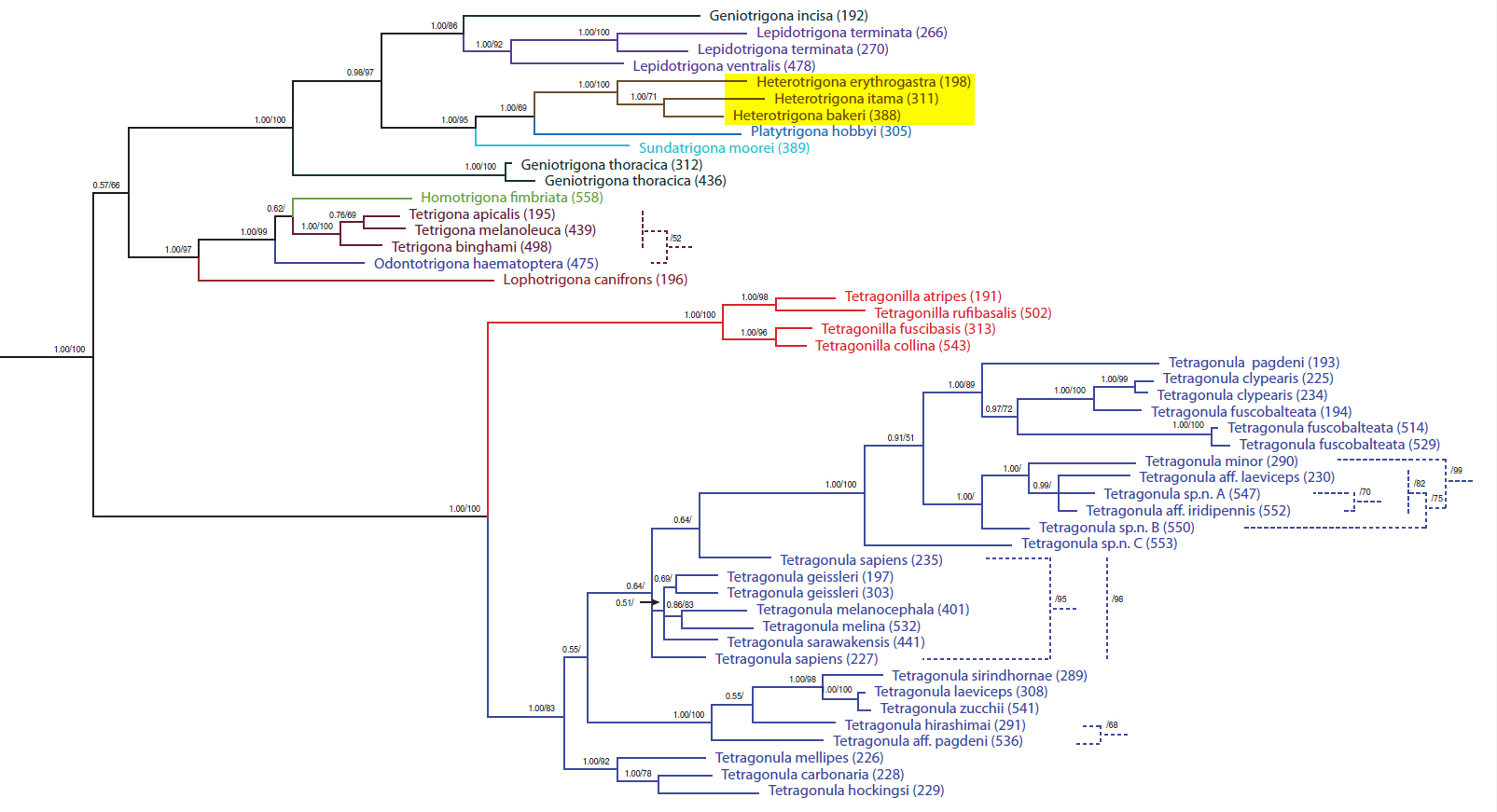
Figure 14. Phylogenetic tree of Old World stingless bees, based on concatenated sequences of five gene fragments: mitochondrial (16S, 28S) and nuclear (opsin, EF1-a, ArgK). Dotted lines represent alternate resolution with maximum-likelihood analysis. The genus Heterotrigona is highlighted in yellow. (Pending permission from author).[33]
The Old World stingless bee genera, historically grouped by similarity of morphological characters, hold up well under the scrutiny of DNA analyses (fig. 14). Heterotrigona, as well as most of the other genera, have all species of the same genus grouped together in the same clade with high bootstrap support. However, when it comes to piecing out relationships between the various genera, support values are not as robust. Also, Geniotrigona incisa and G. thoracica are represented here as not belonging to the same clade, which can lead to the inference that Geniotrigona is not a monophyletic grouping. With only five gene fragments used for this analysis, the status of Geniotrigona is still up for debate, pending further studies utilising more genetic material for analysis.
Phylogenetic relationships in the Apinae
With the growth of genetics in recent decades, there has been a gradual addition of DNA characters to the pool of morphological characters in the classification of stingless bees, as well as bees in general. While there has been general agreement between morphology and DNA approaches for sorting out relationships between species within the stingless bee tribe Meliponini, a fundamental, unresolved conflict remains between the different approaches when it comes to sorting out the classification between tribes in the Apinae.As summed up by Kawakita and colleagues, while morphological[34] [35] [36] , paleontological, and behaviorial[37] data are unified in suggesting that Apini (honeybees) is the sister tribe of Meliponini (stingless bees), genetics data suggests that Bombini (bumble bee) is the sister tribe of Meliponini.[38] An implication of either phylogeny classification is the understanding of the evolution of eusociality in the Apidae. Morphological evidence would imply that eusociality arose once in the evolution of the common ancestor of Apidae tribes, and advanced eusociality, as found in the Apini and Meliponini, arose only once. Genetics data would imply that advanced eusociality in both tribes arose independently of one another, on separate branches of the phylogeny tree.
Type Information return to top
Type locality: SingaporeCollector: Baker, Charles FullerYear collected: 1917-18Type first mentioned in: Cockerell 1918[39] Deposited at: Smithsonian National Museum of Natural History (formerly known as the United States National Museum)References return to top
- ^ Rasmussen, C., & Cameron, S. A. (2010). Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biological Journal of the Linnean Society , 99(1), 206–232.
- ^ Sakagami, S. F., Inoue, T., & Salmah, S. (1990). Stingless Bees of Central Sumatra. In S. F. Sakagami, R. Ohgushi, & D. W. Roubik (Eds.), Natural History of Social Wasps and Bees in Equatorial Sumatra (pp. 125–137). Sapporo, Japan: Hokkaido University Press.
- ^ Schwarz, H. Results of the Oxford University Sarawak (Borneo) expedition: Bornean stingless bees of the genus Trigona (1937). Bulletin of The American Museum of Natural History, 73(3), 281-329.
- ^ Sakai, S., Harrison, R. D., Momose, K., Kuraji, K., Nagamasu, H., Yasunari, T., … Nakashizuka, T. (2006). Irregular droughts trigger mass flowering in aseasonal tropical forests in Asia. American Journal of Botany, 93(8), 1134–1139.
- ^ National Oceanic and Atmospheric Administration (NOAA) Center for Weather and Climate Prediction. (2014, November 4). Historical El Nino/ La Nina episodes (1950-present). Retrieved November 12, 2014, from http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml
- ^ Michener, C. D. (1979). Biogeography of the bees. Annals of the Missouri Botanical Garden , 66(3), 277–347.
- ^ Momose, K., Yumoto, T., Nagamitsu, T., Kato, M., Nagamasu, H., Sakai, S., … Inoue, T. (1998). Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. American Journal of Botany , 85(10), 1477–1501.
- ^ Sakagami, S. F., Yamane, S., & Hambali, G. G. (1983). Nests of some Southeast Asian stingless bees. Bulletin of the Faculty of Education, Ibaraki University (Natural Sciences), 32, 1–21.
- ^ Seeley, T. D., & Morse, R. A. (1976). The nest of the honey bee (Apis mellifera L.). Insectes Sociaux, 23(4), 495–512.
- ^ Inoue, T., Sakagami, S. F., Salmah, S., & Yamane, S. (1984). The process of colony multiplication in the Sumatran stingless bee Trigona (Tetragonula) laeviceps. Biotropica, 16(2), 100–111.
- ^ Winston, M. L. (1987). The Biology of the Honey Bee (p. 281). Cambridge, Massachusetts: Harvard University Press.
- ^ Peters, J. M., Queller, D. C., Imperatriz-Fonseca, V. L., Roubik, D. W., & Strassmann, J. E. (1999). Mate number, kin selection and social conflicts in stingless bees and honeybees. Proceedings of the Royal Society B: Biological Sciences, 266, 379–384.
- ^ Eltz, T. (2001). Ecology of stingless bees (Apidae, Meliponini) in lowland dipterocarp forests in Sabah, Malaysia, and an evaluation of logging impact on populations and communities. Bayerischen Julius-Maximilians Universität Würzburg.
- ^ Moo-Valle, H., Quezada-Euán, J. J. G., & Wenseleers, T. (2001). The effect of food reserves on the production of sexual offspring in the stingless bee Melipona beecheii (Apidae, Meliponini). Insectes Sociaux, 48(4), 398–403.
- ^
Van Schaik, C. P., Fox, E. A., & Sitompul, A. F. (1996). Manufacture and use of tools in wild Sumatran orangutans. Die Naturwissenschaften, 83, 186–188.